
The global pharmaceutical industry is no stranger to major M&A deals, but the current wave reveals a sector adjusting to life after the ‘patent cliff’ and cashing in on tax inversions while they can.
JONATHAN WATSON
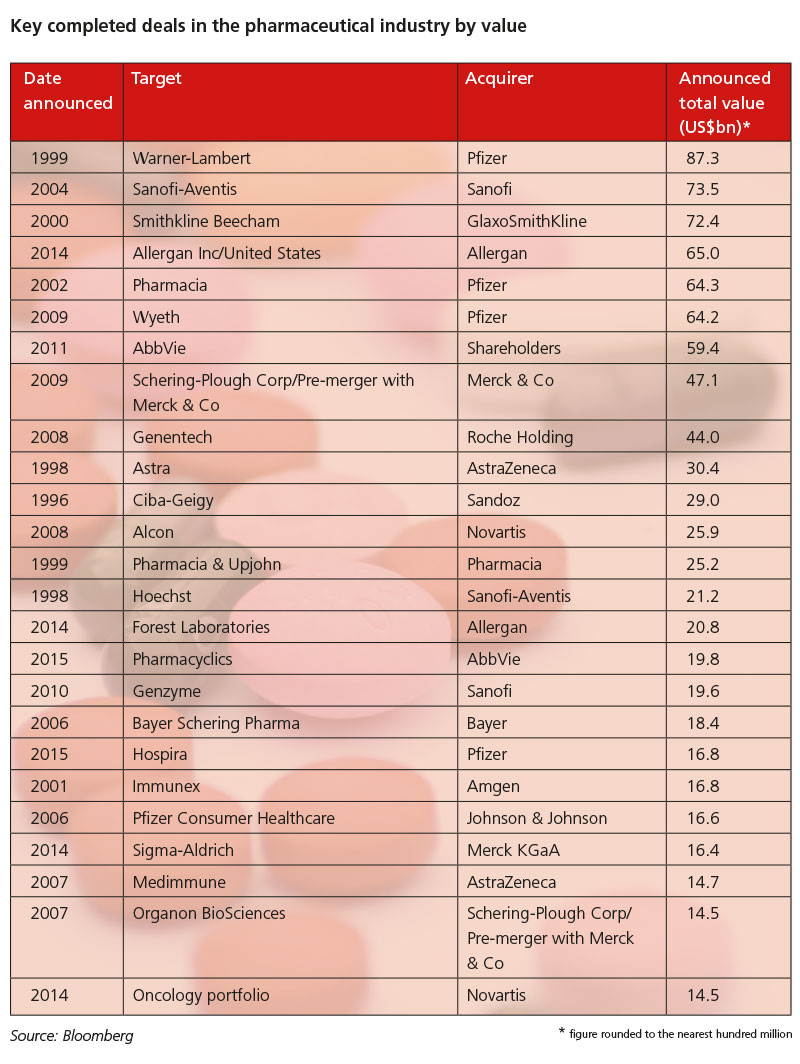
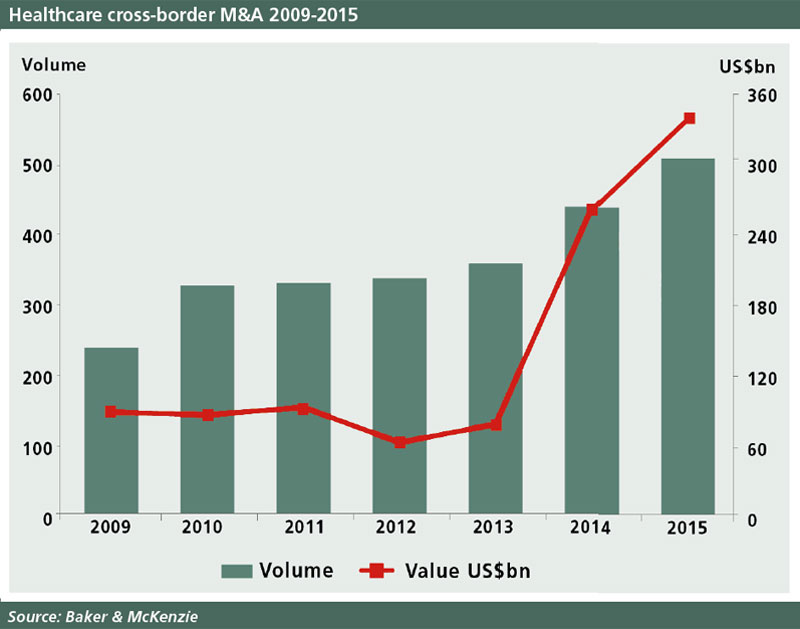
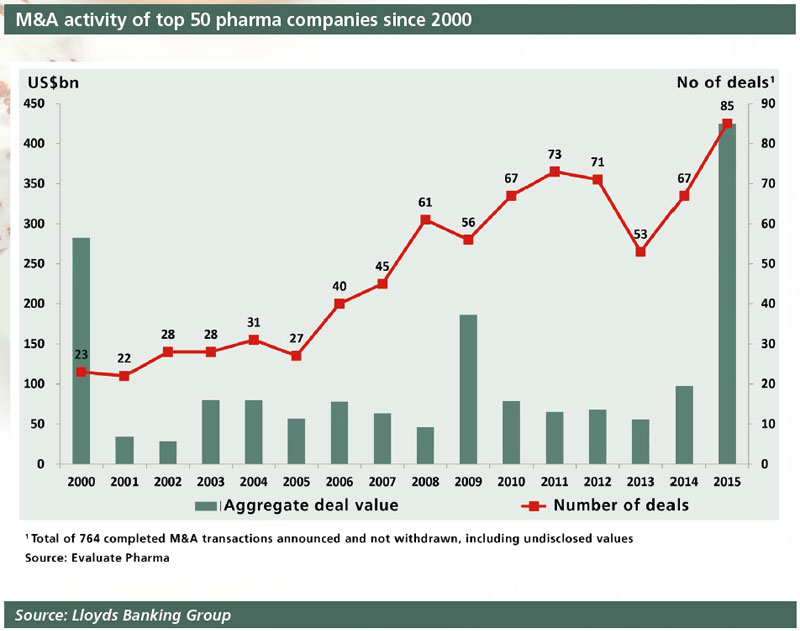
The pharmaceutical industry has been gripped by an M&A frenzy in the last couple of years, with an unprecedented series of mergers, licensing deals and takeovers involving both big pharma and smaller biotechs. Law firm Baker & McKenzie calculated that cross-border pharmaceutical M&A reached $259.7bn in 2014 and $378.9bn in 2015.
US giant Pfizer’s planned acquisition of Allergan for $160bn was expected to be the latest deal, but was recently abandoned amid plans to change US tax laws. Had it gone through, it would have been the biggest M&A in the sector’s history.
‘With pharmaceuticals, the big companies are still seeking to replace drugs that have gone off-patent, so they are retrenching back to core businesses – and we have seen a number of spin-offs and transactions as a result – while exciting innovation in key therapy areas such as oncology are yet to come online,’ says Jane Hobson, chair of Baker & McKenzie’s global pharmaceutical and healthcare industry group.
Surviving the patent cliff
The issue of drugs going off-patent, the so-called ‘patent cliff’, has been a major driver of pharmaceutical deals for some years. When the patents of a company’s leading products are due to expire, it faces the prospect of a sharp decline in revenues, as those products can then be replicated and sold at much cheaper prices by competitors.
‘If pharmaceutical companies don’t have new products in their pipeline, they buy them,’ explains Stephan Rau, a partner at McDermott Will & Emery and Senior Vice-Chair of the IBA Healthcare and Life Sciences Law Committee. ‘Companies are looking at biotech, at smaller companies, and seeking to buy them or to enter into venture capital-style agreements to secure the rights to products so they can get them into their pipelines.’
As part of this process, drug companies have started to focus on particular therapeutic areas and divest from other areas to go back to their core strengths. In 2014, for instance, GlaxoSmithKline and Novartis decided to swap assets worth $20bn. The former has opted to focus on vaccines, while the latter is proving successful with generic oncology drugs and eyecare products.
Generics manufacturers have a different model – low margins but high volume – and are looking to create critical mass
Jane Hobson
Baker & McKenzie
From a funding perspective, this is a good time to close a deal in the pharmaceutical sector. ‘Clearly, there is a willingness from shareholders and debt capital markets to finance such transactions, so there is ample liquidity at the moment and debt is relatively cheap,’ says Philipp Gutzwiller, global head of healthcare at Lloyds Banking Group. ‘At the same time, pharmaceutical companies are hugely cash generative. Even if they gear up for a transaction, they can be leveraged again quite quickly. From a lender perspective, they are a relatively low-risk proposition to support.’
Tax considerations have also been a factor. Under certain conditions, US companies buying a non-US company can switch their tax domicile to the home country of their takeover target, which is why so many drug companies based in low-tax countries like Ireland have been involved in M&As in the past few years. Before it was called off at the start of April 2016, Pfizer’s planned acquisition of Allergan was perhaps the most obvious – and tax campaigners would say the most blatant – example of this.
Ian Read, Pfizer’s chairman and chief executive, said that the company ‘approached this transaction from a position of strength and viewed the potential combination as an accelerator of existing strategies.’ It would have enabled Pfizer ‘to more efficiently allocate our capital around the world,’ he added.
In September 2014, the US Treasury introduced new rules to make such ‘inversions’ more difficult. They did not stop medical device maker Medtronic’s $43bn acquisition of Ireland’s Covidien going ahead, but the US buyer was forced to raise an extra $16bn in debt to finance the merger. A bigger deal, in which AbbVie was due to pay $54bn for Shire (whose operations are mainly in the UK, but whose tax domicile is in Ireland), had to be abandoned. AbbVie paid Shire a break-up fee of $1.6bn and criticised the US Government for moving the goalposts.
It was a recent announcement by the US Treasury that it intends to further tighten up US corporation tax laws to deter these inversions that prompted Pfizer to abandon the Allergan deal, which had been expected to be completed in the second half of 2016.
However, with Sanofi signing two deals in January 2016 with biotech companies worth up to €1.2bn, the current wave of M&A looks set to continue for now. This is partly because large numbers of deals can often generate follow-on deals. ‘Acquirers might have to sell off parts of the companies or products for antitrust reasons, or because large acquisitions quite often include products or businesses that are not core for the acquiring entity and so are sold off relatively quickly after purchase,’ says Hobson.
There is also a good deal of activity among manufacturers of generic drugs (those no longer protected by patents, such as paracetamol). ‘They have a different business model to innovators, low margins but high volume, and are looking to create critical mass,’ adds Hobson. ‘We have seen, for example, a number of Indian generics companies looking for targets in the US, and that again is driving more activity.’
Industry in flux
The pharmaceutical industry is, of course, no stranger to M&As. There have been mega-mergers for some years now, going back to Glaxo Wellcome and SmithKline Beecham in 2000, Pfizer and Pharmacia in 2002, and Pfizer and Wyeth in 2009, to name just a few.
The current wave reflects industry changes that were already underway. ‘The big change was the gradual closedown of the huge drug discovery operations,’ says Patricia Barclay, founder of Edinburgh law firm Bonaccord and Chair of the IBA Healthcare and Life Sciences Law Committee. ‘Some companies sold off their big research units or closed them down. They kept some hi-tech areas they thought had a big future, but we’re seeing some of those closing down now too.’ Large companies also continue to outsource R&D operations, increasing their use of contract research organisations and contract manufacturing.
‘One of the other things we’ve seen over the last couple of years is a change in how the big companies are doing their venturing,’ explains Barclay. In the past, most of the big pharmaceutical companies would set up venture funds of their own for investment purposes. Some only invested in ideas that fell within the interest areas of the parent companies, but others acted like a normal venture fund.
‘Instead of setting up their own venture fund, companies have put money into existing venture funds,’ says Barclay. ‘The quid pro quois that, where the venture fund invests in a company, it gets certain option rights for the technology being developed. That’s quite an interesting deal structure, and we’re seeing more and more like it.’
That can have a downside for some smaller companies, which can end up with a sense of having given away their future at a very early stage. If a company is only a few years away from licensing something out, this may not be a major issue. However, if companies have what they consider a platform technology, and it’s their idea – rather than just a compound they have bought from another company and are developing – they can begin to lose
their enthusiasm.
‘They can become quite disillusioned,’ says Barclay. ‘I think there are issues with that model, especially if the price mechanism and other details are already in place. People are not left with much to work for.’
Orphan diseases
Another feature of current M&A activity is that so-called ‘orphan’ diseases are becoming more of a focus area for pharmaceutical companies. These are diseases that have not been ‘adopted’ by the industry as there is not much of a financial incentive for the private sector to make and market new medications to treat or prevent them. They may be rare diseases (according to US criteria, a disease that affects fewer than 200,000 people), or common diseases that have been largely overlooked by drug developers (such astuberculosis, cholera, typhoidand malaria)because they are far more common in developing countries than in the
developed world.
Regulators are now prepared to grant an exclusivity period once a product is approved for a certain disease, and this prevents potential competitors from getting any new products approved in the same area. ‘Not only do pharmaceutical companies have patent protection, they also have seven to ten years of exclusivity, depending on the jurisdiction,’ says Gutzwiller. ‘During that time, the companies can interact closely with the community of patients, parents, carers, payers and so on affected by the disease. They effectively own that small market.’
This means drug prices can be relatively high. ‘Orphan diseases are becoming more attractive,’ says Rau. ‘There are rules which offer companies advantages in terms
of reimbursement.’
Shire is one company pursuing this strategy. It gets an estimated 45 per cent of its revenue from rare disease treatments – a percentage that is set to rise to around 65 per cent with the purchase of Baxalta, agreed in January 2016. Shire is to pay $32bn for the company in a deal that its chief executive Flemming Ornskov said would create the leading specialist producer of therapies for rare diseases, with projected revenues of $20bn by 2020.
‘Companies that specialise in this sector are able to successfully build capabilities that can be leveraged across other rare diseases and adjacent therapeutic areas,’ said a Shire spokesperson. ‘These include experience with natural history studies, registries, diagnostics, market access and a deep understanding of how to manage small patient populations.’
This knowledge of the population is vital if Shire’s strategy is to succeed. ‘Over time, these organisations are able to scale and optimise the rare disease model, ultimately developing and delivering innovative medicines to a broader range of patients with high unmet medical need,’ the spokesperson added.
Sanofi is another company interested in this area. David Meeker, head of Sanofi’s Genzyme specialist care business, stated at the end of February that rare disease assets were among the French group’s potential targets as it looked for growth. Acquisitions were possible ‘up to the size of Genzyme’, which Sanofi bought for $20bn in 2011, he said.
Deal making dynamics
There is a risk that the fast pace of merger activity could have a destabilising effect on the companies involved, particularly in light of staffing changes. ‘With some of the big mergers, there are boasts about cost cutting, but they might cut swathes of people without really having the time to go into it in as much detail as they should,’ says Barclay. ‘They then find that people whose skills are needed have gone and they have to be hired back.’
In many cases, too many regulatory affairs staff tend to be dispensed with too soon. ‘When a merger is badly handled, you can be left with a regulatory team that has too much work to do and a lot of old products belonging to the company that’s been acquired which are based on technologies they know absolutely nothing about,’ explains Barclay. ‘Many regulatory people have decided to give up their jobs after going through one merger too many. It’s not good for morale or for a company’s productivity.’
This can be especially problematic in the pharmaceuticals sector because so many drugs are so heavily regulated. Transferring the regulatory requirements across to the buyer can create significant issues. ‘There are a lot of transitional service arrangements [TSAs] in place between buyers and sellers,’ says Hobson. ‘These can last for quite some time, so the two companies can end up being highly dependent on each other for many years after the disposal.’
In some cases, there are then disposals of disposals – meaning transactions are taking place before the TSAs have come to an end. That can create a good deal of contractual complexity, adds Hobson. ‘From a lawyer’s perspective, you have to be very careful when drafting a TSA because you have to keep in mind there could be another transaction while the TSA is still in force.’
There are those who believe that the pharmaceutical industry has had a relatively easy ride. ‘It’s never been through a financial crisis in its own right that has flushed out the excess layers of management and complex procedures that may lead to a suboptimal business model,’ said one industry source.
Shire and Baxalta, for example, have similar sales figures, but Baxalta has almost twice as many employees. There can be good reasons for that, but Shire perhaps provides more of a blueprint for what a pharma company should look like, the source said. ‘As for the big companies like GlaxoSmithKline, Pfizer and Sanofi, they have been big for a very long time, but have also been very successful, so the hard questions have never really been asked. They haven’t faced that much pressure to redefine their processes or streamline their organisations.’
This could be a problem as the industry becomes more complex from a legal perspective, suggests Gutzwiller. ‘M&A deals have their own legal contracts that need to be negotiated. We’re seeing more earnout elements being included, where the sellers must “earn” part of the purchase price based on the performance of the business following the acquisition,’ he says. ‘Companies are also having to make sure they can defend themselves against lawsuits from shareholder activists who claim the board is selling the company below its value.’
We’re seeing more earnout elements being included, where the sellers must ‘earn’ part of the purchase price based on the performance of the business following the acquisition
Philipp Gutzwiller
Lloyds Banking Group
The question of who owns medical data is also set to become more complicated. Is it patients, doctors or pharmaceutical companies? What can be done with the data, and who can gain access to it? ‘Insurance companies are always looking to select good risks and deselect bad risks, so if there is a way of finding out that someone is more likely to become ill in a certain way, they want to know about it,’ says Gutzwiller. ‘We are only in the foothills of that; it is likely to be determined in the courts.’
In addition, many companies are seeking to acquire or develop biosimilars, which are generic impersonations – although not identical copies – of biotechnologically derived drugs. Lawyers are likely to find themselves involved in more and more discussions about this. ‘There might, for instance, be a biotech product which is a protein that cannot be patented per se, but things around it can be patented, such as the production process,’ explains Gutzwiller. ‘That makes it more difficult for anyone to copy, both from a technical and increasingly from a legal perspective.’ A company may come up with a slightly better version of a biotech drug, but legal hurdles mean it cannot be marketed.
As biosimilars threaten the sales of biotech companies such as Amgen and Genentech (now part of Swiss conglomerate Roche), they are fighting hard to defend their patch. Recently, for example, Sandoz (part of Novartis) called on the US Supreme Court to review a lower court ruling that biosimilar makers should wait an additional six months after regulatory approval before bringing their product to market. This is part of an extensive legal battle over Sandoz’s Zarxio drug, which came to market as a biosimilar version of Amgen’s Neupogen.
The pharmaceutical industry often gets accused of putting profits before patients, and there are longstanding controversies over the design of trials and use of drug development data. Some see pharmaceutical companies’ culture of M&A as further evidence that they only care about – and seek to acquire – medicines they can profit from, rather than those that are likely to be the most beneficial.
This debate won’t be resolved anytime soon, but Hobson believes the implications of the M&A activity for healthcare overall are positive. ‘When drug companies focus around particular therapeutic areas, putting all their “eggs in one basket”, and traditional competitors align to collaborate or form joint ventures over particular indications,
that is hopefully going to improve the output – and ultimately improve what is available to patients in need.’
Jonathan Watson is a freelance journalist and can be contacted on jwatson1@gmail.com